From Analytics to Testing of Packaging – GBA Pharma GmbH is Expanding their Testing Capabilities
GBA Pharma GmbH in Ulm, Germany is one of three GMP-certified and FDA-inspected laboratories of the GBA Group Pharma. More than 52 employees are dedicated to providing analytical services for domestic and international customers,whom they support throughout every step of the product life cycle. The lab recently expanded their portfolio in the area of syringe testing with the zwickiLine materials testing machine from ZwickRoell.
While purely analytical tests on drugs may have been sufficient in the past, there have been tremendous changes in the last several years. Testing on primary packaging materials and the combination of drug and primary packaging (referred to as a combination product) has continued to gain significance.A wide range of ISO standards, including ISO 11040 for prefilled syringes and various chapters in the US Pharmacopeia (USP) such as USP <382> or USP <1382> require physical and functional testing of combination products.
Testing the glide force of prefilled syringes to ISO 11040-4 Annex E/G2
Based on this development in testing requirements and increasing inquiries from customers, GBA Pharma expanded their testing capabilities accordingly. The requirements on testing systems, particularly for companies in the regulatory environment are extremely high. In addition, as a testing service provider they must be able to carry out a wide variety of tests to meet diverse customer needs. Within the scope of quality control and acceptance processes for pharmaceutical and biotechnology products, the GBA Pharma laboratory also offers regulation compliant handling of powerful active ingredients. For now, the focus is on two tests. With the high demand on Corona vaccines, there is an increased requirement for penetration tests with injection needles on vials. In addition, tests are performed to determine the breakaway and glide forces on prefilled syringes to ISO 11040-4 Annex E and G2, as well as USP <1382>.

zwickiLine – the ideal testing machine
GBA Pharma chose ZwickRoell as their partner and decided on a zwickiLine Z0.5 testing system. The required tests can be performed with standard test tools and pre-defined standard test programs. This significantly reduces the time required to set up the system before the test. The modular design allows for quick changes in the test arrangement. Additional test tasks can be easily carried out without a lot of effort.Mr. Christian Mangold, site manager in Ulm listed a few other deciding factors:“I have had great experiences with ZwickRoell testing machines in the past. The testXpert III testing software meets every requirement in accordance with FDA 21 CFR Part 11.The highly knowledgeable ZwickRoell qualification team supported us throughout the joint computer system validation process. As a service provider in the GMP Pharma environment, we confidently rely on ZwickRoell as our experienced supplier. They fully support us in meeting the requirements for data integrity in materials testing in our field.”
Cleanroom capability up to ISO Class 6 and Class 5 to DIN EN ISO 14644-1
Erik Berndt, Industry Manager for Medical/Pharma at ZwickRoell has also been following these increasing standards requirements:“To help meet the strict GMP requirements on pharmaceutical companies, ZwickRoell provides support through compliant materials testing machines and testing software, as well as comprehensive documentation.” “We are also seeing an increasing need for the use of testing solutions in cleanroom conditions. We meet this need with our zwickiLine machine series, which fulfills cleanroom capabilities up to ISO Class 6 and optionally Class 5 to DIN EN ISO 14644-1,” continued Berndt.“The local proximity to GBA Pharma helps us support customers with their test tasks on newly developed drugs, especially for cancer treatment, as approval and experience with handling cytostatic agents are available here.”
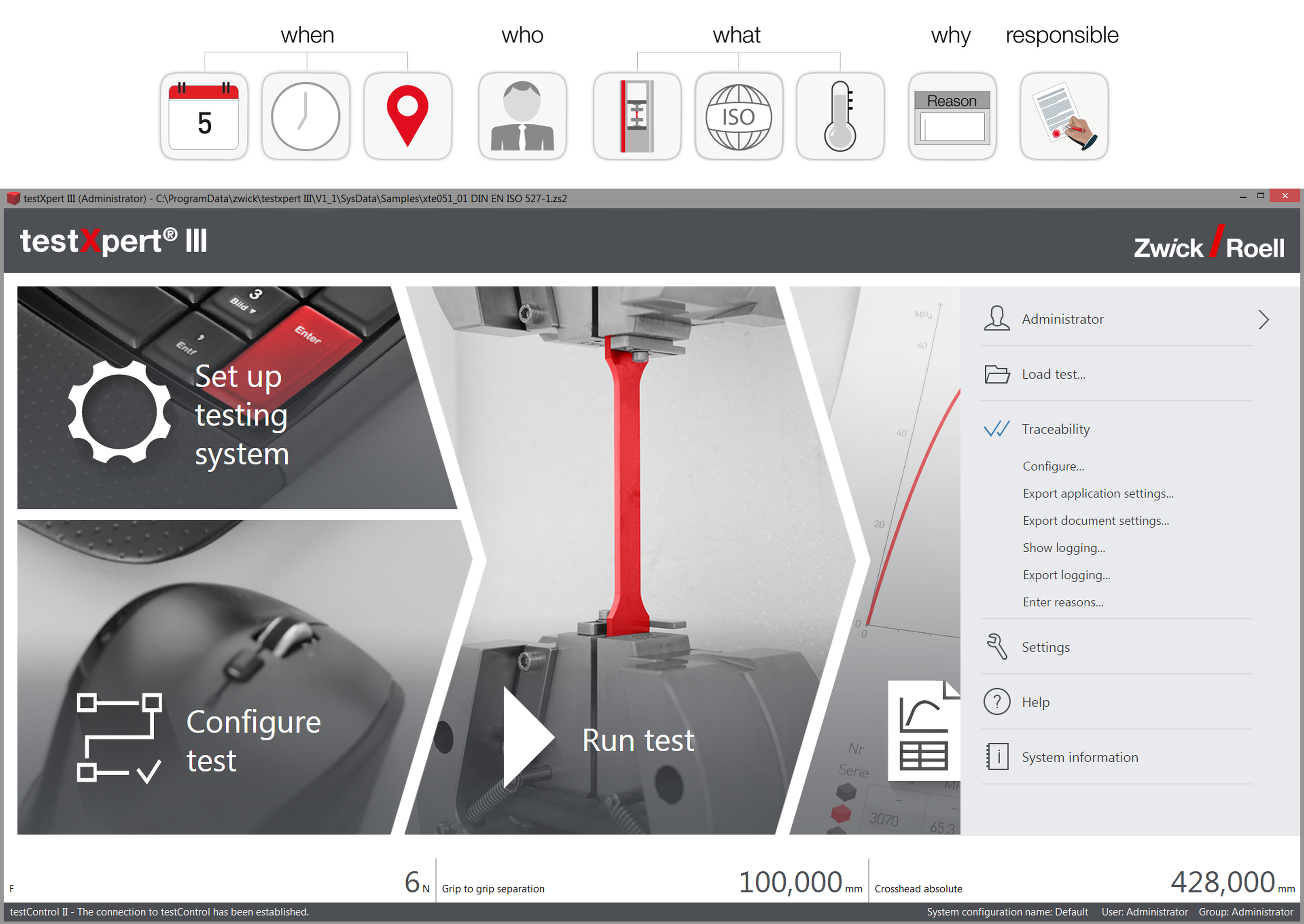
testXpert III convinces customers
“I have had great experiences with ZwickRoell testing machines in the past. The testXpert III testing software meets every requirement in accordance with FDA 21 CFR Part 11.The highly experienced ZwickRoell qualification team stood by our side throughout the computer system validation process. As a service provider for GMP Pharma, we confidently rely on ZwickRoell as our experienced supplier. They fully support us in meeting the requirements for data integrity in materials testing in our field,” said Christian Mangold, Site Manager GBA Pharma.