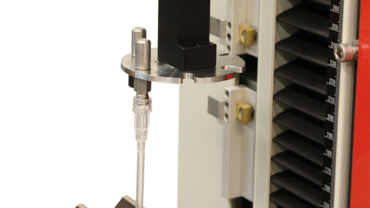
DIN EN ISO 10555 with ZwickRoell
- To determine the insertion and connecting forces of catheter tube systems in accordance with DIN EN ISO 10555, each individual connector must be loaded in tension until failure. For this, a wide variety of connecting diameters are required.
- The 8050 self-aligning tension grip has a rotating self-locking disc with various opening widths used to test numerous connectors.
- Specimen Grips are used for repeatable gripping of the opposing side of the catheter. The specimen grips are closed by means of a foot pedal, leaving both hands free to insert the specimen. The closing force can be set steplessly with the pneumatic control unit, and the low overall height of the specimen grips facilitates optimal use of the materials testing machine's test area.
- In addition, ZwickRoell offers a wide range of jaws for the most diverse types of applications.
Advantages / features of our testing equipment:
- Disc-locking feature enables tool-free changes between test environments.
- The generous specimen grip height enables testing of large specimens (for example, needle pull-out tests on syringes).
- The specimen grip type 8050 can be used with any materials testing machine via ZwickRoell's standard connector system.
- Easy changeover to additional rotating discs.
Traceable, tamper-proof test results in accordance with FDA 21 CFR Part 11
Traceable tamper-proof test results
- Ever-increasing demands are placed on software used in the medical and pharmaceutical industries to document the traceability of completed actions.
- With the traceability option, testXpert III enables logging of all actions and changes before, during and after the test, making test results and the documentation traceable and protecting them from manipulation.
- Integrated user management and functions such as electronic records and electronic signature ensure that test results are always protected from tampering.
- Together with the organizational measures and procedure instructions that apply to the individual companies themselves, the requirements of FDA in 21 CFR Part 11 are fulfilled.
- ZwickRoell also offers a qualification service package (DQ/IQ/OQ) for validation support.
- testXpert III logs all test and system related actions and settings and can therefore always answer the question “When does who do what, why and who is responsible?”
Learn more about the testXpert III
Traceability option.