Standard guide for radial loading of balloon-expandable and self-expanding vascular stents
Along with obtaining accurate material characteristic values, determining the radial compression strength is the most important test for stents. They must exert a radial force that is sufficient to ensure that the stent remains in the narrowed spot and prevents constricting of the blood vessels. ASTM F3067 describes the radial compression testing of balloon-expanding and self-expanding stents.
ZwickRoell offers the zwickiLine series materials testing machine—a testing solution equipped with a 37 °C chamber to simulate tests at body temperature. The radial compression test fixture by Blockwise, which measures the radial force, is specifically designed to test stents and is available in various diameters and lengths. It simulates the pressure placed by the artery on the stent and the return force exerted by the segmentally arranged wedge jaws that generate a uniformly distributed surface pressing. The stent is inserted, compressed radially up to a minimum target diameter, and then released. The testXpert III testing software supports the sequence by measuring the values. It not only compensates for possible self-deformations, but also takes into account the very slight frictional and inertial forces that arise with a ZwickRoell machine during measurement.
Non-contact strain measurement on wires and stent struts
To simulate stent systems, detailed material characteristics are required. Along with tests for the entire system, components such as single wires and stent struts are often tested. This also includes the tensile strength and strain at break, as well as the minimum yield strength. It defines the force at which a material under a single-axis tensile load demonstrates no permanent deformation. More efficient and above all more accurate is strain measurement using an extensometer. The probability of error is much smaller since measurements are taken directly at the specimen and therefore outside the force flow.
ZwickRoell offers a wide portfolio of materials testing machines and numerous extensometers. Choosing the most suitable extensometer for the application is essential. The difference is whether or not the extensometer makes contact with the specimen during measurement. Clip-on extensometers are a cost-effective way to measure values, but can falsify measurements because of the direct contact it makes, or they can damage the specimen. This is the danger with specimens made of thin wire. The weight of the clip-on extensometer alone could lead to bending of the specimen. Furthermore, there is a risk that the knife edges slip and damage the wire. A safe and simultaneously accurate way to measure is to use a non-contact extensometer.
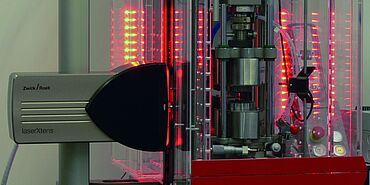
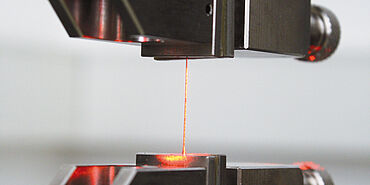
ZwickRoell's non-contact extensometer, laserXtens, is designed for tensile, compression, and flexure tests on various materials. It creates a speckle pattern on the surface of the specimen, which is recorded by a full image digital camera. This pattern creates a virtual gauge mark on the specimen, whose movement under load is tracked with a special correlation algorithm. The evaluation of two sequential images shows the strain of the specimen with a resolution of less than 0.15 μm. This non-contact strain measurement is also used on stents made of nitinol to obtain accurate material characteristics for the FEM simulation—from the beginning of elastic deformation until strain at break.
ZwickRoell offers the non-contact extensometer, laserXtens, in various models. laserXtens Compact is a single-camera measuring system specifically designed for testing short and thin specimens. It meets all requirements set forth by Class 0.5 of ISO 9513 (Class B2 of ASTM E83) and can be used with all table-top and floor-standing AllroundLine testing machines from ZwickRoell. An even more accurate model, which is able to capture the smallest variances, is the laserXtens Compact HP. It achieves a resolution of 0.04 μm.
With the laserXtens, the operator needs only a few seconds to set the various gauge lengths. In addition, it is easy to mount and dismantle, and it is combined with largely automated test sequences, which significantly reduce the amount of resources and time needed for testing. This in turn increases the quality of the tests, because subjective influences are minimized, which is particularly efficient and useful in series testing or for tests with integrated production chains. Measuring specimens in temperature chambers are also possible.
Fatigue strength under compression and torsion load
Another development from ZwickRoell tests the fatigue strength of a stent under a periodically changing load. To investigate the durability of stents ZwickRoell has developed a fixture that allows up to 30 stents to be accommodated simultaneously. This fixture, with an electric torsion drive (1 Nm), is used in combination with an HC 10 servo-hydraulic testing machine (10 kN) and allows both separate and superimposed loading of the stents with compression and torsion (5 Hz at ± 60°). The gripped area of the stents can also be provided with a fluid bath to enable testing under physiological conditions.
Traceable, tamper-proof test results in accordance with FDA 21 CFR Part 11
- Ever-increasing demands are placed on software used in the medical and pharmaceutical industries to document the traceability of completed actions.
- With the traceability option, testXpert III enables logging of all actions and changes before, during and after the test, making test results and the documentation traceable and protecting them from manipulation.
- Integrated user management and functions such as electronic records and electronic signature ensure that test results are always protected from tampering.
- Together with the organizational measures and procedure instructions that apply to the individual companies themselves, the requirements of FDA in 21 CFR Part 11 are fulfilled.
- ZwickRoell also offers a qualification service package (DQ/IQ/OQ) for validation support.
- testXpert III logs all test and system related actions and settings and can therefore always answer the question “When does who do what, why and who is responsible?”
Learn more about the testXpert III
Traceability option.