ISO 11193 / DIN EN 455-2 Testing of rubber gloves
Disposable medical gloves protect during operations, examinations, and nursing activities. The most important requirement is that they are impermeable to air, fluids, and microorganisms. They are manufactured by immersing molds in solutions of natural rubber or latex mixtures. The immersion and drying processes are repeated until the required thickness is achieved, after which the gloves are vulcanized in curing ovens.
Objective of the standard Video Specimen preparation Running a test Testing systems Accessory
Purpose of the test
So that they do not tear when being pulled on or while in use, the gloves must display sufficiently elastic behavior. Examples of stresses to which they are subjected include hooking or holding pointed or sharp tools, foreign bodies or instruments against the gloves.
To ensure the required functional safety and to ensure that the single-use gloves offer and maintain an adequate level of protection, they are tested according to ISO 11193-1, ISO 11193-2 and DIN EN 455-2.
SO 11193 / DIN EN 455-2 Specimen preparation
For the test according to ISO 11193, three dumbbells are taken from the palm or back of the hand of a glove.
For the test according to DIN EN 455-2, 13 gloves are taken from each batch, from which one dumbbell specimen is to be blanked from the inside of the glove, the back of the hand or the glove cuffs. The direction of the cut should be parallel to the longitudinal axis and structured areas should be avoided.
The specimens must not have any folds or creases.
ZwickRoell offers suitable cutting presses for the standard-compliant production of specimens.
Test to ISO 11193-1 & -2 and DIN EN 455-2
In addition to determining the dimensions, water resistance and powder limit values, ISO 11193-1 and -2 describe the determination of tensile stress-strain properties such as tear strength and strain at break on examination gloves both before and after accelerated aging.
ISO 11193-1 describes two different types of rubber gloves:
- Type 1: Gloves made mainly of natural rubber latex.
- Type 2: Gloves made of other rubber solutions (such as nitrile, polychloroprene, SBK, etc.)
ISO11193-2 focuses on rubber gloves made of PVC
The tensile stress-strain properties must be determined according to ISO 37 in a tensile test.
When tested according to DIN EN 455-2, the dumbbells are clamped in a materials testing machine and pulled at a test speed of 500 mm/min. until break. The force at break is determined in Newtons. The median of the 13 results must meet the minimum values defined in the standard.
Testing systems for tests to ISO 11193 and DIN EN 455-2
The zwickiLine universal testing machines, space-saving for small test loads and ProLine with a test area from 1050 mm to 1450 mm, enable 100% standard-compliant testing of rubber gloves:
- The very large work area of the load frame offers sufficient space for the most flexible materials with high elongation.
- For a high specimen throughput, the machine returns to the starting position very quickly after the test.
- Start testing immediately: Easy-to-learn, intuitive operation and the advantages of automated functions reduce training effort and ensure consistent measurement accuracy
- Minimized operator influence through defined starting position and predefined machine configurations
- The testXpert testing software meets the requirements of FDA 21 CFR Part 11 with its traceability function
Specimen grips for testing of rubber gloves
ZwickRoell offers various types of specimen grips, from manual mechanical specimen grips to comfortable pneumatically controlled systems, each with suitable grip inserts.
- Pneumatic grips: The gripping force is generated by pneumatic actuators and can be operated with a hand or foot control. Gripping force independent of the tensile force ensures a constant test speed over the course of the entire test sequence.
- Pincer grips: double actuator grip. The gripping force is increased proportionally by the pincer principle as the tensile force increases. This ensures automatic retightening for specimens prone to shrinkage.
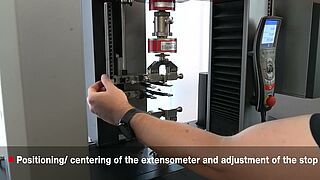
Suitable extensometers
During the tensile test on rubber gloves, not only the middle part of the test specimen stretches, but also the shoulder. That is why the measuring accuracy of the extensometer is crucial. The requirements of the standards can be met either by extensometers that are mechanically attached directly to the specimen or by optical, non-contact extensometers. ZwickRoell offers suitable extensometers with high measurement accuracy – from cost-efficient, manual extensometers to fully automatic systems, completely without operator influence and with maximum reproducibility of test results.