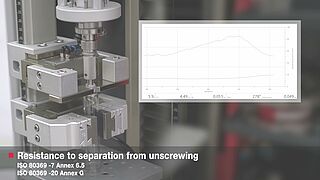
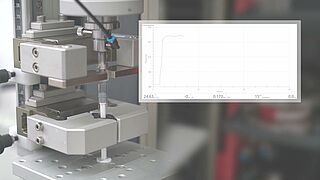
ISO 80369-7 and ISO 80369-20: Testing of Luer system/Luer lock connectors
Based on the ISO 80369-7 and ISO 80369-20 standards, various tests are used to test a Luer system or Luer lock connector for stability—ZwickRoell offers the right testing system for the job.
ISO 80369 for the testing of Luer systems/Luer locks replaces the standards ISO 594-1 and ISO 594-2 (first edition in 1986 as well as the second edition from 1998).
ISO 80369 content Definition Luer/Luer-Lock connection Videos Automation Testing systems Testing software Medical brochure Customer application example
Contents of the standard ISO 80369
ISO 80369 defines the testing for small-bore connectors for liquids and gases in healthcare applications. Part 1 defines the general requirements. Parts 2 through 7 describe the different connectors. Part 20 addresses the test methods to be applied.
- ISO 80369-1: General requirements
- ISO 80369-2: Connectors for respiratory and propulsion gas applications
- ISO 80369-3: Connectors for enteral applications
- ISO 80369-4: Connectors for urethral and urological applications
- ISO 80369-5: Connectors for limb cuff inflation applications
- ISO 80369-6: Connectors for neuraxial applications
- ISO 80369-7: Connectors for intravascular or hypodermic applications
- ISO 80369-20: General test methods
What is a Luer system or Luer lock connector?
- The Luer cone is a standardized connector system used in tube systems in the medical industry. It is used with cannulas, syringes, catheters, three-way stopcocks, and infusion tubes. The seal is achieved through a conical fitting, called a Luer cone. The Luer lock connector also has a threaded sleeve to achieve a tight connection so fluids cannot escape.
- For quality control of these components, a materials testing machine with superimposed torsion drive is used.
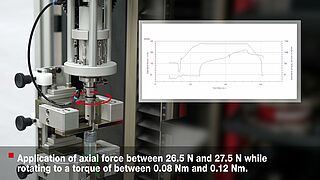
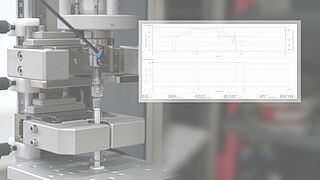
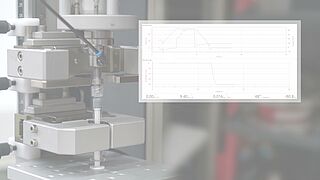
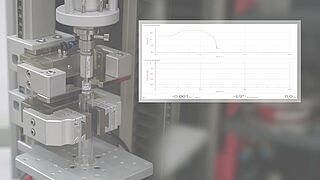
- Based on the ISO 80369 standard, various tests are used to test a Luer system or Luer lock connector for stability. It is possible to easily determine torques under static axial load with the superimposed axial/torsion drive of a zwickiLine materials testing machine.
- With the integrated compression fixture, the seal integrity tests required by the standard can be performed.
Video on ISO 80369: Testing of small bore Luer connectors
Luer lock testing: Revisions to ISO 80396 require reliable test results to ensure data integrity.
The semi-automatic test on small bore Luer connectors to ISO 80369-7 and ISO 80369-20 is performed using a zwickiLine testing machine with torsion drive.
Individual tests of Luer lock connections to ISO 80369
ISO 80369 – Automated testing of Luer lock connectors
When dealing with large production batches, a testing process that saves time and resources is often important. ZwickRoell provides a simple solution for automated testing of Luer lock connectors.
The testing assistant roboTest N supports the user in performing the test to ISO 80369. Typically, 30 Luer Lock connectors can be tested automatically with one magazine filling. The testing assistant transports the specimen from the magazine to the Luer Lock testing system and positions it in the specimen grips. Here, the thread as well as the seal tightness of the Luer Lock connector are tested. The testing assistant roboTest N then removes the specimen from the machine and returns it to the magazine.
The system can be easily and flexibly adapted to changing test requirements without requiring any special programming knowledge. For all robotic testing systems, the autoEdition3 automation software is used for information input and control.
Traceable, tamper-proof test results in accordance with FDA 21 CFR Part 11
- Ever-increasing demands are placed on software used in the medical and pharmaceutical industries to document the traceability of completed actions.
- With the traceability option, testXpert III enables logging of all actions and changes before, during and after the test, making test results and the documentation traceable and protecting them from manipulation.
- Integrated user management and functions such as electronic records and electronic signature ensure that test results are always protected from tampering.
- Together with the organizational measures and procedure instructions that apply to the individual companies themselves, the requirements of FDA in 21 CFR Part 11 are fulfilled.
- ZwickRoell also offers a qualification service package (DQ/IQ/OQ) for validation support.
- testXpert III logs all test and system related actions and settings and can therefore always answer the question “When does who do what, why and who is responsible?”
Learn more about the testXpert III
Traceability option.