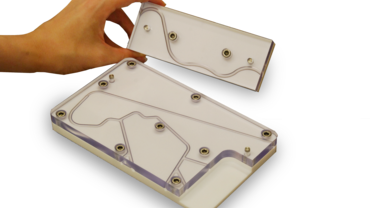
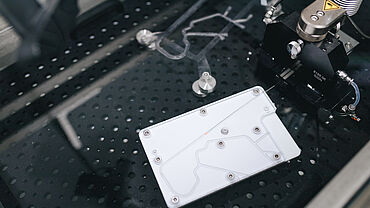
Horizontal testing on catheter systems
Catheters are used for many reasons, including transporting microinstruments integrated in a patient's body. They enable surgeons to make surgical procedures as minimally invasive as possible. A catheter can also help administer medicine directly to the infected area of the body or facilitate the use of stents to keep blood vessels open.
In the continuing development of these catheter systems or guide wires, efforts are being made to reduce the coefficient of friction and the breakaway torque. The ZwickRoell horizontal AllroundLine testing machine enables determination of shear forces in a simulated catheter insertion with very high accuracy. The frictional behavior of the catheter is measured by pushing it through an artificial artery. This is known as the tortuous path. The test is carried out in a horizontal orientation to simulate the physiological status of the patient during surgery.
ZwickRoell has developed a system specifically for this application that controls both the crosshead of the materials testing machine and a special automated specimen grip. The horizontal AllroundLine testing machine provides space for 3D models and fluid baths above and below the main test axis. In a typical test, the machine pushes the catheter into the artificial aorta for a predefined distance. The specimen is then released and the crosshead returns to its initial position. The pneumatic grip re-closes and the crosshead again moves in the direction of the test. This sequence repeats itself until the catheter is completely inserted in the artificial artery. The fully automated test method makes it easy to test artificial aortas of various lengths. The following results can be determined with the machine software:
- Insertion force measurement: measures the force used to advance through the introducer sheath
- Track force: measures the force required to advance the catheter, guide wire, or another minimally invasive instrument through the artificial artery
- Push efficiency: uses the proximal and distal load cell to measure the amount of force the distal tip of the product sees when a known force is being applied to the product on the proximal end
- Guide wire movement: measures the force needed to advance a guide wire through a catheter, a guide catheter, or a similar minimally invasive instrument
- Flexibility: measures a catheter tip's ability to track over a specified bend in a guide wire, such as 90 degrees
- Guide wire and catheter lubricity track measurement: comparative test using the track test data to determine if coatings have an affect on the force required to advance product through an artificial artery
The results can be determined with a high level of accuracy. The extremely stiff load frame with digital control and drive systems is able to ensure that forces measured during the test originate from the sample under test and not from within the machine itself. The machine's control system has such a high resolution that it is able to position the crosshead of the machine to less than 1µm, and read forces to an accuracy of better than 0.5 % down to values of less than 0.1 mN.
Traceable, tamper-proof test results in accordance with FDA 21 CFR Part 11
- Ever-increasing demands are placed on software used in the medical and pharmaceutical industries to document the traceability of completed actions.
- With the traceability option, testXpert III enables logging of all actions and changes before, during and after the test, making test results and the documentation traceable and protecting them from manipulation.
- Integrated user management and functions such as electronic records and electronic signature ensure that test results are always protected from tampering.
- Together with the organizational measures and procedure instructions that apply to the individual companies themselves, the requirements of FDA in 21 CFR Part 11 are fulfilled.
- ZwickRoell also offers a qualification service package (DQ/IQ/OQ) for validation support.
- testXpert III logs all test and system related actions and settings and can therefore always answer the question “When does who do what, why and who is responsible?”
Learn more about the testXpert III
Traceability option.