ISO 11608-1, ISO 11608-2, ISO 11608-3 Testing of pen injectors / insulin pens
The standards ISO 11608-1, ISO 11608-2 and ISO 11608-3 describe the requirements and test methods for tests on pen injectors and insulin pens. These tests are particularly performed for quality assurance purposes. They are used to measure actuation force, dosage accuracy, plunger movement, among others.
Overview ISO 11608 USP 1382/382 Test requirements Dosing accuracy Pen Power Pack Connected medical devices Automation Videos Testing systems Testing software Medical brochure
Overview ISO 11608
ISO 11608: “Needle-based injection systems for medical use - requirements and test methods”
- ISO 11608-1: Needle-based injection systems
- ISO 11608-2: Needles
- ISO 11608-3:Finished containers (referenced in USP <1382> and USP <382>
- ISO 11608-4: Needle-based injection systems containing electronics
- ISO 11608-5: Autoinjectors
- ISO 11608-6: On body delivery systems
United States Pharmacopeia Chapter USP 1382 and USP 382
Furthermore, USP (United States Pharmacopeia) Chapter 1382> and 382> reference the ISO 11608-3 standard in particular.
Based on ISO 11608-3, the requirements on functional proficiency tests for breakaway force, glide force, and leak tightness are described here.
- USP 1382> This chapter provides information and guidance to assist in evaluating the functional proficiency of elastomeric components as part of packaging/drug delivery systems intended for parenteral dosage forms.
- USP 382> This chapter addresses functional proficiency requirements for packaging/drug delivery systems intended for parenteral dosage forms.
ISO 11608-1, ISO 11608-2, ISO 11608-3 with ZwickRoell
- The most important requirements when testing medical products are the reproducibility of results and the minimization of operator influences. To meet these requirements, ZwickRoell has developed automated systems for testing pen injectors (for example, for insulin). Insulin therapy normally involves the subcutaneous injection of insulin by means of prefilled syringes or insulin pens. These pens are similar to writing pens and are filled with insulin cartridges. Single-use pens are disposed of after the contents of the cartridge are dispensed, whereas reusable pens can be used year after year. For quality assurance tests on insulin pens and carpules, the standardDIN EN ISO 11608-1, DIN EN ISO 11608-2 and DIN EN ISO 11608-3 applies.
- ZwickRoell testing systems and test fixtures also fulfill the requirements of USP (United States Pharmacopeia) Chapter <1382> and <382>.
Dose accuracy to DIN EN ISO 11608-1
A testing system consisting of a zwickiLine testing machine with additional torsion drive is used to test insulin pens. This testing system can measure various pen functions, such as the dosage setting, actuation force, glide force, and specified dosage in one continuous process. You can modify and combine the test methods of both test axes to suit your testing needs.
Testing the pen power pack
The testing system can perform these tests to check the pen's mechanical function without the drug. Along with force and torque, it measures the activation force with the utmost precision. When testing filled pens, an integrated high-resolution scale is used to measure the dosing accuracy to DIN EN ISO 11608-1.
Test: networked medical devices (connectivity)
Intelligent, networked medical devices are on trend, and for many good reasons: On the one hand, they allow patients to better visualize the use of the medical device; on the other hand, they facilitate advanced data collection and evaluation possibilities for medical personnel. As a result, medical devices are increasingly including electronic components that, in combination with suitable technology such as Bluetooth Low Energy (BLE), near-field communication (NFC) or WLAN, can connect to corresponding cell phone apps and exchange data.
Examples of networked medical devices
In addition to wearables, such as watches that are already transmitting the wearer’s heartbeat, oxygen saturation and other body function data, inhalers, smart pillboxes/medication boxes, insulin pens and other drug delivery products also feature this type of technology. Or the blood glucose meter on a patient's upper arm. This device measures the current value and sends it to the insulin app, which uses the value to calculate the insulin volume to be injected and transmits suggestions to the patient. The insulin pen in turn reports the administered amount and the time of administration to the app, which records and analyses the patient-critical data. Networked medical devices must comply with applicable data protection and cyber security regulations, such as those defined in IEC 62304, ISO 13485, MDR 2017/745, GDPR or NIST SP 1800-30.
Connected medical devices:testing solutions from ZwickRoell
ZwickRoell offers you testing solutions, which in addition to mechanical tests for various product characteristics of medical devices, also provides corresponding hardware and software components to capture the transferred data. These can be used to, for example, record, decode and evaluate the data sent by the networked medical device. Data integrity is therefore verified by comparing the data obtained during the test with the data sent.
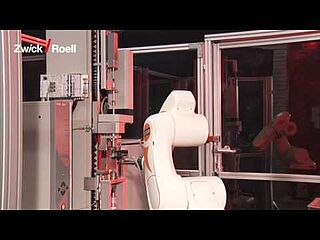
Automated testing of insulin pens and pen injectors to ISO 11608
When testing insulin pens or pen injectors, a time and resource efficient test process if often important. A zwickiLine materials testing machine with integrated torsion drive, combined with an automated testing systems, ensures reliable and cost-effective test results .
The testing assistant roboTest N supports the user with simple applications. With one magazine filling, you can typically automatically test 30 insulin pens or pen injectors. The testing assistant roboTest N can be easily adapted to changing test requirements with a high level of flexibility, without requiring special programming knowledge.
The robotic testing system roboTest R allows you to run fully automated tests on different functions of the insulin pen. You can, for example, measure the dosage setting, actuation force, glide force, and specified dosage in one continuous process. You can modify and combine the test methods of both test axes to suit your testing needs.
It uses the automation software autoEdition3 to remove insulin pens from the magazine, feed them into the testing machine, and start the test. This minimizes the possibility of inaccurate test results due to operator influence. Our solution makes the test process significantly more efficient thanks to increased specimen throughput. It is also possible to manually test specimens at any time.
The testXpert testing software together with the Expanded Traceability option to FDA 21 CFR Part 11 make it possible to create documentation for the testing process that is complete and tamper proof.
Videos on testing pen injectors / insulin pens to ISO 11608
Traceable, tamper-proof test results in accordance with FDA 21 CFR Part 11
- Ever-increasing demands are placed on software used in the medical and pharmaceutical industries to document the traceability of completed actions.
- With the traceability option, testXpert III enables logging of all actions and changes before, during and after the test, making test results and the documentation traceable and protecting them from manipulation.
- Integrated user management and functions such as electronic records and electronic signature ensure that test results are always protected from tampering.
- Together with the organizational measures and procedure instructions that apply to the individual companies themselves, the requirements of FDA in 21 CFR Part 11 are fulfilled.
- ZwickRoell also offers a qualification service package (DQ/IQ/OQ) for validation support.
- testXpert III logs all test and system related actions and settings and can therefore always answer the question “When does who do what, why and who is responsible?”
Learn more about the testXpert III
Traceability option.